Abstract
Background: Phosphatidylserine (PS), a cell membrane phospholipid present in the inner layer of the plasma membrane, is expressed on the surface of red blood cells (RBCs) from individuals with congenital and acquired hemoglobinopathies including sickle cell disease (SCD) and thalassemia. These cells are involved in the genesis of several pathobiologic processes including perturbed hemostasis. Based on the amount of cell surface PS, Yasin et al (Blood, 2003) have grouped PS+ RBCs into low level PS type-1 (PS1) and high level PS type-2 (PS2) cells. In SCD, the majority of transferrin receptor-positive (CD71+) stress reticulocytes (retics) are PS1+, whereas the majority of PS+ dense cells are PS2+and are specific to SCD. PS exposure on retics in subjects with Hemoglobinopathies appears to be physiologically relevant and may play a role in retic maturation. In contrast, dense cells may play a role in the pathogenesis of several SCD-related complications including skin ulcers, pulmonary hypertension and renal disease.
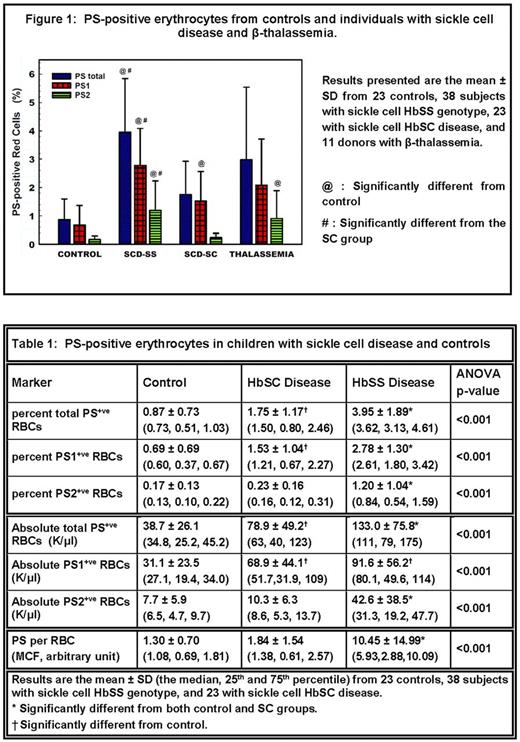
Aim: To assess whether PS2+ red cells are present in other hemoglobinopathies besides SCD, and to evaluate which PS fraction associates with hemolysis and hemostatic activation.
Methods: Sixty-one children with SCD (38 HbSS and 23 HbSC, age range 1.5 to 22 years), 23 age- and race-matched controls, and 11 with β-thalassemia were evaluated for the presence of PS+ RBCs by flow cytometry using RBCs labeled with annexin V-FITC (PS marker) and anti-glycophorin A-PE (red cell marker). The data was analyzed for PS1+ and PS2+ RBCs, and also for PS per RBC assessed as mean cell fluorescence (MCF). PS levels among groups and between groups were compared for statistical differences. Representative markers of hemostatic perturbation (D-dimer) and hemolysis (LDH) were measured, and relationships with PS1+ and PS2+ RBCs evaluated. In in vitro assays, osmotic fragility of PS2+ RBCs was tested. In a subset of SCD and controls, PS+ RBCs were further characterized using red cells labeled with annexin V-FITC and anti-CD71-PE (a stress retic marker) to delineate PS1+ and PS2+ stress retics and non-retics. Data was analyzed in HbSS based on stress retic levels (low vs high).
Results: In contrast to Yasin, we found that PS2 cells were not specific for HbSS, since they were present at similar levels in subjects with β-thalassemia (Figure 1). Both PS1+ and PS2+ red cells (percent and absolute number) as also PS/cell were significantly increased in HbSS compared to HbSC and controls (Table 1). In HbSS, PS2+ RBCs correlated positively with D-dimer (p<0.02). No D-dimer correlation was found with PS1+ RBCs. Neither PS1+ nor PS2+ RBCs in HbSC correlated with D-dimer. Additional analysis demonstrated that PS2+ RBCs in HbSS correlated with the hemolytic marker LDH (p<0.001). In a complementary in vitro assay, we found that PS2+ RBCs were osmotically fragile and readily hemolyze. To confirm the previous demonstration by Yasin of minimal to no PS2+ stress retics in HbSS, we further undertook an analysis of this biomarker in HbSS subjects with low vs high stress retics. We demonstrate that while PS2+ stress retics were present minimally in subjects with low stress retics, they were significantly elevated in individuals with high stress retics (median PS2+ stress retics of 827 and 3269 /μl blood, p<0.03). A positive correlation was also noted between PS2+ stress retics and CD71+ RBCs (r=0.50, p<0.01, n=26).
Conclusion: We demonstrate that PS2+ RBCs, which are elevated in HbSS compared to HbSC and controls, are not specific to SCD as they were also present at similarly elevated levels in β-thalassemia. We also demonstrate the presence of PS2+ stress retics in HbSS subjects with high retic levels. Previous studies in HbSS implicated PS+ RBCs in modulating hemostasis. Our results delineate that PS2+ red cells are the PS sub-group involved in this activation. We further demonstrate that PS2+ red cells are associated with elevated LDH levels. These cells are osmotically fragile releasing multiple inflammatory mediators including heme, which is a modulator of thrombin generation. These findings taken together suggest a pathophysiologic role for PS2+ red cells linking hemolysis with hemostasis in hemoglobinopathies including HbSS and β-thalassemia.
Acknowledgments: supported by NIH grants P60HL62148, U54HL70585 and P20GM109021
Data was collected at St Christopher's Hospital for Children and Thomas Jefferson University, Philadelphia PA.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.